|
Background: |
The DnaJ family is one of the largest of all the chaperone families and has evolved with diverse cellular localization and functions. The presence of the J domain defines a protein as a member of the DnaJ family. DnaJ heat shock induced proteins are from the bacterium Escherichia coli and are under the control of the htpR regulatory protein. The DnaJ proteins play a critical role in the HSP 70 chaperone machine by interacting with HSP 70 to stimulate ATP hydrolysis. The proteins contain cysteine rich regions that are composed of zinc fingers that form a peptide binding domain responsible for the chaperone function. DnaJ proteins are important mediators of proteolysis and are involved in the regulation of protein degradation, exocytosis and endocytosis. DnaJA4 (DnaJ homolog subfamily A member 4) is a SREBP-regulated chaperone that is thought to regulate the cholesterol biosynthesis pathway. |
|
Applications: |
ELISA, WB, IHC |
|
Name of antibody: |
DNAJA4 |
|
Immunogen: |
Fusion protein of human DNAJA4 |
|
Full name: |
DnaJ heat shock protein family (Hsp40) member A4 |
|
Synonyms: |
MST104; MSTP104; PRO1472 |
|
SwissProt: |
Q8WW22 |
|
ELISA Recommended dilution: |
5000-10000 |
|
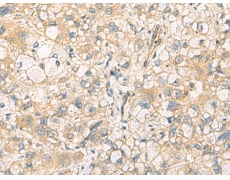
IHC positive control: |
Human liver cancer and Human tonsil |
|
IHC Recommend dilution: |
50-200 |
|
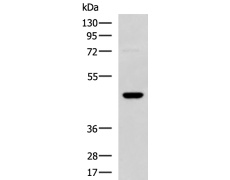
WB Predicted band size: |
45 kDa |
|
WB Positive control: |
TM4 cell lysate |
|
WB Recommended dilution: |
500-2000 |



 購物車
購物車 幫助
幫助
 021-54845833/15800441009
021-54845833/15800441009